Rishena’s implantable vagus nerve stimulator (VNS) officially approved for marketing
From: News Date: 2020-02-14 13:28
Rishena’s implantable vagus nerve stimulator (VNS) officially approved for marketing
On February 5, 2020, CFDA approved the market registration application of the implantable vagus nerve stimulator (VNS) developed by Changzhou Rishena Medical Devices Co., Ltd.
?
This means that patients with epilepsy in China will have more opportunities to enjoy better treatment and services at lower medical costs.
?
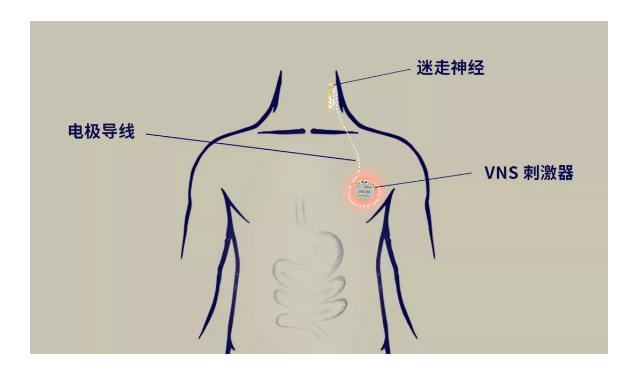
The implanted vagus nerve stimulator (VNS) stimulates the neck vagus nerve by sending electrical pulse signals, which is transmitted to the brain of patients with epilepsy and suppresses seizures. It is mainly suitable for adjuvant treatment of patients with refractory epilepsy.
?
Epilepsy is currently one of the most common diseases of the nervous system diseases, causing severe damage to patients' physical and mental health. There are approximately 9 million patients with epilepsy in China, of which 20% -30% are refractory epilepsy, and seizures cannot be effectively controlled through regular drug treatment.
?
Starting from 2010, Tsinghua University Microelectronics Institute has teamed up with partner hospitals to carry out experimental research on implantable neurostimulators. After years of animal experiments and equipment research and development, it led to the incubation of Changzhou Rishena Medical Devices Co., Ltd., which was established in 2013.
?
After years of efforts by the Rishena team, in 2015, the vagus nerve stimulator (VNS), one of the implantable neurostimulator products, was officially launched for clinical trial. It was led by Wang Yuping, director of the Department of Neurology, Xuanwu Hospital, Capital Medical University and jointly participated by the epilepsy center of Renji Hospital affiliated to Shanghai Jiaotong University Medical College, the epilepsy center of the First Affiliated Hospital of the General Hospital of the Chinese People's Liberation Army, the Department of Neurosurgery of the 306 Hospital of the People's Liberation Army, and the Neurology Department of Beijing Friendship Hospital affiliated to Capital Medical University.
?
Clinical studies have shown that VNS can significantly reduce the frequency of seizures, with an overall effective rate of 53% -63% (effectiveness: 50% or more reduction in the number of seizures); during clinical periods, various clinical centers carefully adjust parameters to VNS for patients with refractory epilepsy; and many useful VNS treatment methods for patients with refractory epilepsy have been explored.
?
The VNS system developed by Rishena Medical has a remote control function, which allows the patient's program controller to be controlled by the doctor's parameters at home, reducing the trouble of patients traveling to and from the hospital multiple times; by establishing a control center, it ensures that the control is more efficient and portable. Rishena VNS is manufactured with precision technology, ultra-low power consumption, long service life, soft and firm electrode leads, and high comfort.
?
The implanted Vagus Nerve Stimulator (VNS) developed by Rishena Medical is officially approved for marketing, which will bring hope to more patients with epilepsy.?
Rishena Medical has been focusing on the development and production of active medical devices for many years. The clinical trials of the deep thermocoagulation brain electrode (SEEG) also have been completed; and the clinical trials of the spinal cord electrical stimulation system (SCS) have been launched; the company will continue to innovate and continue to make breakthroughs, so that the patients can enjoy higher quality medical treatment and services at a lower cost.


